As a GMP-certified pharmaceutical company, Tcmages has established a rigorous and comprehensive quality control system throughout its production process.
The company has adopted the infrared (IR) fingerprint quality standard system to monitor the overall manufacturing process of FCG granules to ensure that all sourced raw materials and intermediate and finished products strictly comply with the controlling indicator standard so that the required premium quality is maintained for all products.
Just as human fingerprints are unique to each individual person, so infrared spectroscopy provides a spectral fingerprint that uniquely identifies a particular chemical compound. Since no two compounds have the same infrared spectrum, identity of the sample can therefore be confirmed by comparison of this region to a known spectrum.
Every materia medica has a different fingerprint depending on its locality and species and the methods employed in its processing — this is its specific feature. The fingerprint of a particular materia medica originating from the same locality and species and processed correctly according to the same method is consistent across different samples — its specific feature can therefore be replicated. Comparison of samples against benchmark standards ensures that raw materials come from traditionally authentic areas of cultivation and that the best-quality plant species are accurately identified and correctly processed.
Quality control – the basis for corporate development and a major contribution to the granule industry and society in general
FCG full composition granules are produced from Chinese materia medica prepared according to regulatory standards. More than 32 million of our products have been dispensed to date without any quality incidents or reports of adverse reactions among patients, a major testament to the establishment of a comprehensive quality control system and our total commitment to product safety.
The main problem for the Chinese herbal granule sector is quality control and since maintenance of granule quality stability and uniformity is a sector-wide problem, it also has to be addressed by Tcmages. Since its establishment, when undertaking research and development and designing production techniques, the company has always prioritized its quality control system. More than ten years of unremitting efforts enabled a comprehensive quality control system to be established, laying a solid foundation for the development of the company and making a major contribution to the production of high-quality pharmaceutical products. Our product classification system, procurement bidding system, supplier assessment system, integrated IR fingerprint quality control system, annual quality feedback system and a complete quality management organization chart and internal control system are integral components of our overall quality control system.
Our digital online IR quality supervision system functions throughout the product manufacturing process and is incorporated in the overall corporate quality control system. For the first time in the industry, a fully integrated product, technical, manufacturing and quality control approach is provided to managers and consumers. This is the biggest contribution that Tcmages has made to the herbal granule and prepared herbal medicine industry.
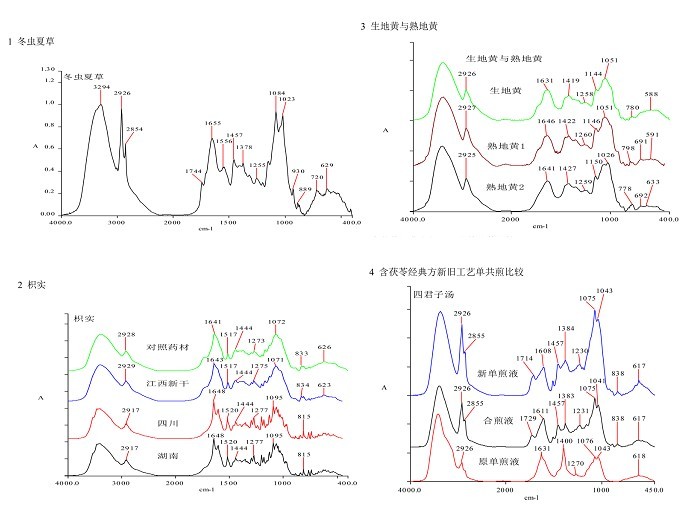
Dong Chong Xia Cao (cordyceps)
Sheng Di Huang (dried rehmannia root) and Shu Di Huang (prepared rehmannia root)
Comparison between new and old processes for decocting Fu Ling (poria) singly or together when used in a classical formula
Zhi Shi (Immature bitter orange)